MATRIX PATCH TECHNOLOGY
Corplex® is the subject of several US and international patents. These fundamentally cover a broad range of adhesive systems and bioavailability enhancement approaches for small molecules.

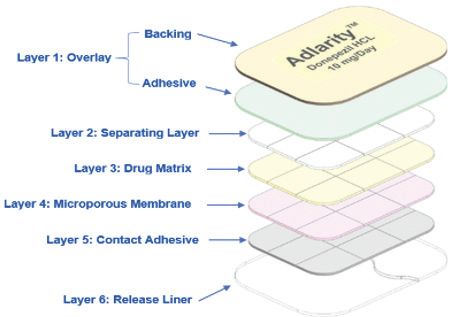
Our FDA-approved 7-day continuous delivery patch containing the widely prescribed drug Donepezil for the treatment of Alzheimer’s Disease, was developed via the Corplex® platform.
The innovation driver for this product is the in-patch conversion process to transform the salt form of drug to a higher bioavailability, free-base form of donepezil that results in higher levels of drug being absorbed across the skin. In addition, the transdermal delivery of donepezil potentially results in fewer GI-related adverse events relative to oral delivery of the drug.
Key features
CORPLEX® TECHNOLOGY
- Range of adhesive properties for aqueous and lipid delivery
- Improved drug bioavailability and high drug loading
- Excellent adhesion to both wet and dry surfaces
- Flexibility with natural skin movement
- Continuous delivery to 7 days from a single patch
- Patent protection
PARTNERING WITH US
FOR SUCCESS
We can provide assistance in the following areas depending upon your marketing needs:
- Innovative, patented products for specialty pharmaceutical companies
- Life Cycle Management expertise for new chemical entities
- Bioequivalent products for the lower-cost, generic market
- Unique OTC consumer products that provide market differentiation
Please reach out to our business development
team for further information:

Kevin Ostrander MS, MBA
- Chief Business Officer
- E. kevin.ostrander@coriumintl.com
- T. +1 (908) 240-4486

Dwayne Sharpe
- Director of Business Development
- E. dwayne.sharpe@coriumintl.com
- T. +1 (636) 734-5123


